We have to have the same number of atoms of each species in both sides of the reaction.
Lets call:
a: mols of H3PO4
b: mols of P2O5
c: mols of H2O
Balance for hydrogen H:
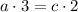
Balance for Phosphorus P:
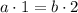
Balance for Oxygen O:
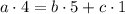
We can express a b in function of c, and then solve backwards:

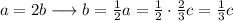
This system has infinite solutions. The third equation gives an identity, so we must fix a value for c and then calculate the others.
As b and c are "thirds" of c, we select c=3.
Then:
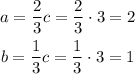
As b=1, we know that is the lowest possible combination of integers:
Answer:
- 2 mols of H3PO4
- 1 mol of P2O5
- 3 mols of H2O