1) Balance the chemical equation.
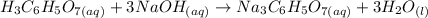
2) List the known and unknown quantities.
Sample: citric acid.
Volume: 20.0 mL = 0.0200 L
Molarity: unknown.
Base: NaOH.
Volume: 18.4 mL = 0.0184 L
Molarity: 0.100 M
3) Moles of NaOH that reacted.
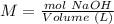
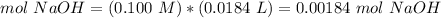
4) Convert moles of NaOH to moles of H3C6H5O7.
The molar ratio between NaOH and H3C6H5O7 is 3 mol NaOH: 1 mol H3C6H5O7.
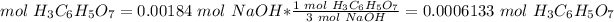
0.0006133 mol H3C6H5O7 reacted.
5) Find the molarity.
Sample: citric acid.
Volume: 0.0200 L
Molarity: 0.0006133 mol.
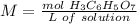
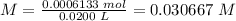
The concentration of citric acid (H3C6H5O7) is 0.0307 M.
.