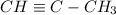The exercises refer to hydrocarbons. Depending on the type of bond, it will be the termination of the name of the molecule.
For a single bond: End with the suffix -ane
For double bond: Ends in -ene
For triple bond: Ends in -yne
Let's look at each particular case
a) Heptene
Hept- means 7 carbons, so we have a compound with 7 carbons and one double bond. The formula will be:
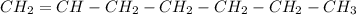
c)Hexane
6 carbons with a simple bond
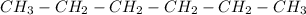
d)Pentane
5 carbons with a simple bond
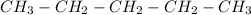
e)Propyne
3 carbons with a triple bond