Answer:
Exothermic reaction
Explanations
The given diagram is the diagram showing the change in the heat of the reaction of a substance.
If the enthalpy change is negative, the reaction is exothermic showing that the heat is given out to the surroundings but the enthalpy change is positive, the reaction is exothermic showing that the heat is absorbed from the surrounding.
From the diagram, the energy of the product is C while the energy of the reactant is A.. Since the energy of the product is lower than that of the reactant, hence the change of enthalpy will be expressed as:
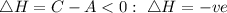
Since the enthalpy change is negative, the reaction is exothermic showing that the heat is given out to the surroundings.