Step 1 - Understanding the formula for molality
Molality is a concentratio defined as the quotient between the number of moles of solute and the mass of solvent.
We can state it mathematically as:
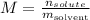
Step 2 - Setting the data in the equation
The exercise has given us all the needed data: the number of moles of solute (lactose) is 1.5 whereas the mass of solvent (water) is 0.5kg. Therefore:
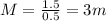
The molality of this solution will be 3 moles/kg or 3m.