They ask us to prepare an HCl solution, they give us the molarity (1.5M) and liters of the solution (0.40L) to find the grams we need.
Molarity is defined by the following equation:
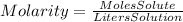
We can clear the moles of solute from the equation:
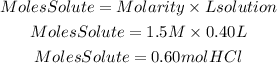
Now, we find the grams of HCl multiplying the grams by the molar mass of HCl. The molar mass of HCl is 36.458g/mol.
The grams of HCl needed will be:
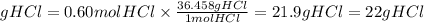
We will need 22 grams of Hcl to prepare the solution
Answer: Second option. 22 grams