Take into account that the electric power supplied by the heating coil is given by:
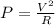
where,
V: voltage of the operatio of the heating coil = 210V
R: resistance = 20Ω
Replace the previous values of V and R into the expression for P and simplify:
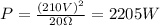
The previos value is equivalent to the energy supplied by the heating coil per second. This energy is aboserved by the water and then the heat absorved by the water in a time t, to increase the temperature from T1 to T2, can be written as follow:
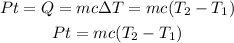
where, for the given situation you have:
m: mass of the water = 200kg
c: specific heat of water = 4186 J/(kg°C)
T2: final temperature of water = 80°C
T1: initial temperature = 15°C
t: time = ?
Now, if you solve for t, replace the values of the other parameters and simplify, you obtain:
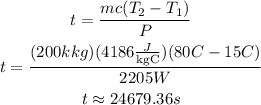
The previous result is in seconds. In hours you obtain:
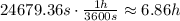
Hence, it is necessary about 6.86 hours to increase the temperature of 200 kg of water 15°C to 80°C