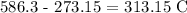Answer:
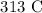
Step-by-step explanation:
Here, we want to find the missing temperature value
According to the pressure law, temperature and pressure are directly proportional to each other
What that means is that as temperature increases, pressure increases, and as temperature decreases, pressure decreases
Thus, for a given mass of gas, we have the following equation representing the relationship between its final and initial state
Mathematically, we have that as:
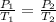
Where:
P1 is initial pressure which is 2 atm
T1 is the initial temperature which is 20 + 273.15 = 293.15 K (we have to convert to the absolute temperature scale when calculating
P2 is the final pressure which is 4 atm
T2 is the final temperature which is ?
Substituting the values, we have it that:
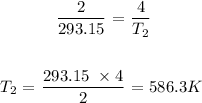
Now, we simply convert this to Kelvin by subtracting 273.15 K
Mathematically, we have that as: