Explanations:
Mole ratios in chemical processes are important to compare the number of moles in a balanced equation. The effect of unbalanced ratio will lead to the presence of leftover reactants.
These mole ratios are used as conversion factors between the reactants and the product. They are crucial in determining the limiting and the excess reactant in a chemical reaction.
The mole ratio is important in material balance of the components that take part in any unit operation or unit processes. For instance they can be used to calculate the amount of feed required for an operation.
For instance, in the reaction of Zinc and hydrochloric acid as shown:
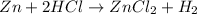
For instance, if the number of moles of HCl in the reaction is 6moles and that of Zinc is 10moles, the moles of hydrogen gas produced will be 3 moles since 2 moles of HCl produces 1 mole of hydrogen gas according to stoichiometry.
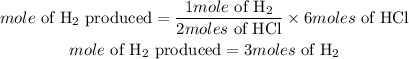
Note that the limiting reactant in the reaction above is hydrochloric acid which was used to predict the moles of the products. HCl is the limiting reactant because the number of moles of HCl is lower than that of Zinc. Zinc will therefore serve as the excess reactant in this case.
The limiting reactant/reagent is the reactant that will be completely consumed during the chemical processes. It is used to predict the mass or mole of product that is produced. The absence of the limiting reactant will limits the reaction from continuing.
The excess reagent on the other hand are reagent that are not used up on completion of the reaction. This reactant can help to increase percentage yields of a chemical process.