Answer:
73.02 grams
Explanations:
Given the following parameter
Atom of hydrogen = 9.42x10^24 atoms
From the given compound, for every 9 moles of hydrogen, there are 3 moles of nitrogen, hence the atoms of nitrogen present in the compound will be:
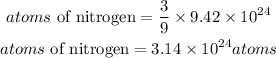
Convert atoms to moles
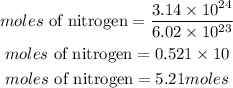
Convert moles of nitrogen to mass
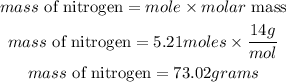
Hence the mass of nitrogen contained in the sample is 73.02 grams