Answer:
Part A: the pressure of the gas is 869.78 torr.
Part B: 3075.4K (2802.25 °C) is needed to reach the pressure.
Step-by-step explanation:
Part A:
1st) The given information in the excecise is:
- P1: 775 torr
- T1: 34°C (307.15K)
- P2: this is what we have to calculate.
- T2: 72°C (345.15 K)
- Volume: constant.
2nd) To calculate the final pressure, it is necessary to use the Gay-Lussac's Law and replace the values:
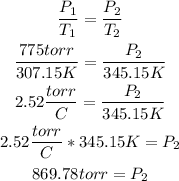
Finally, the new pressure of the gas is 869.78 torr.
Part B:
1st) The given information in this case is:
- P1: 775 torr
- T1: 34°C (307.15K)
- P2: 7750 torr
- T2: this is what we have to calculate.
- Volume: constant.
2nd) To the final temperatue, we can also use the Gay-Lussac's Law and replace the values:
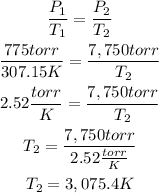
Finally, 3075.4K (2802.25 °C) is needed to reach the pressure.