Before starting to do any calculations we must verify that the reaction is balanced. To do this we count the atoms of each element on both sides of the reaction.
We have:
Cu - 2 atoms
Ag - 2 atoms
N - 2 atoms
O - 6 atoms
The reaction is balanced. So we can proceed.
By stoichiometry, one mole of copper (Cu) will produce 2 moles of silver (Ag). So if we have 50 moles of Copper will be produced 100 moles of silver.
To find the mass of silver we will use the molar mass:
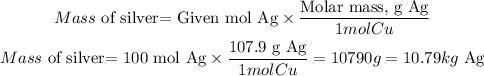
The mass of silver will be 10.79 kg of Ag