ANSWER
The number of mole of the solute is 0.0145 mol
Step-by-step explanation
Given that;
The volume of the solution is 73.97mL
The concentration of the solution is 0.1955M
Follow the steps below to find the number of moles of the solute
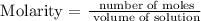
Convert the volume of the solution to Liters
Recall, 1mL = 0.001L
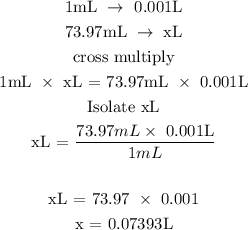
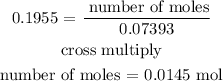
Therefore, the number of mole of the solute is 0.0145 mol