Answer:
HNO2: acid.
SO3 (2-): base.
NO2 (-): conjugate base.
HSO3 (-): conjugate acid.
Step-by-step explanation:
First, we have to understand the concept of Brønsted-Lowry acid-base: An acid-base reaction, according to the Brønsted-Lowry definition, is a transfer of a proton from one molecule or ion to another.
A conjugate acid is the particle produced when a base accepts a proton.
A conjugate base is the particle produced when an acid donates a proton.
Let's see the reaction (6):
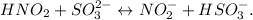
You can note that the acid would be HNO2 (its name says it all: nitrous acid), and therefore, SO3 (2-) would be the base.
Based on the conjugate acid and conjugate base: HSO3 (-) would be the conjugate acid because SO3 (2-) is accepting the proton (H (+)) from HNO2, and the NO2 (-) would be the conjugate base because NO2 (-) is the result of donating a proton from HNO2.