Answer
The final volume, V₂ the gas occupies = 8.81 L
Step-by-step explanation
Given data:
Initial temperature, T₁ = 31 °C = 31 +273 K = 304 K
Initial volume, V₁ = 7.74 L
Final temperature, T₂ = 73 °C = 73 + 273 K = 346 K
Note: The pressure is kept constant.
What to find:
The final volume, V₂ the gas occupies.
Step-by-step solution
Using Charle's law:
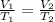
To get V₂, substitute the given parameters into the equation above:

The final volume, V₂ the gas occupies is 8.81 L