Use the following formula for the amount of heat absorbed:
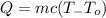
where m is the mass, c is the specific heat of water, To is the initial temperature and T is the final temperature.
Take into account that the amount of heat absorbed by water at 5°C comes from the water at 80°C. Then, you can write:
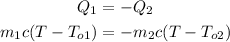
where,
m1 = 5kg
To1 = 5°C
m2 = 3kg
To2 = 80°C
You can cancel specific heat c in the last equation, solve for T, replace the values of the other parameters and simplify, as follow:
![undefined]()