To answer this question we have to use the following equation:
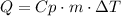
Where Q is the heat applied or released, Cp is the specific heat capacity, m is the mass and ΔT is the difference in temperature. Replace for the given values to find Q:
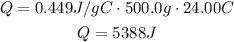
It means that to raise the iron skillet temperature of 24.00°C, 5388J have to be applied.