Firstly, we need to write the equation for the process.
We start with hydrogen gas, H₂, and nitrpgen gas, N₂, and end with NH₃, so the unbalanced reaction is:
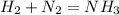
To balance it, we can add a coefficient of 2 to NH₃ so that N gets balanced and then we will need to add a coefficient of 3 to H₂ so H gets balanced:
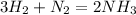
With the balanced reaction, we will need the molar mass of each component, which we can calculate using the molar masses of the atoms H and N:
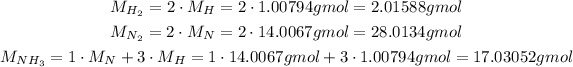
Now, we need to convert the masses of nitrogen gas and hydrogen gas to number of moles:
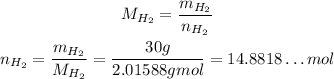
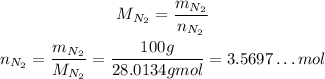
Now, we need to find which of the two is the limiting reactant, that is, which we have less considering the ratio they react.
Each 1 mol of N₂ that react will need 3 mol of H₂, so if all the 3.5697... mol of N₂ react, than we will need
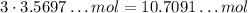
10.7091... mol of H₂. Since we have 14.8818 mol of H₂, we have excess of H₂, which means that N₂ is the limiting reactant.
Since H₂ is the limiting reactant, the most that we can produce of NH₃ is the corresponding of 3.5697... mol of reacting N₂.
From the equation again, we can see that each mol of N₂ that reacts will produce 2 mol of NH₃, so if all the limiting 3.5697... mol react, we will get:
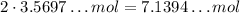
7.1397... mol of NH₃, so this is the maximum number of moles we can get of NH₃:
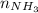
Using the molar mass of NH₃, we can convert this to mass:
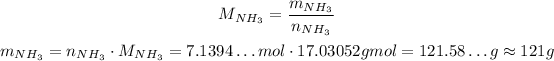
So, the maximum mass of NH₃ you can produce with the given masses of reactant is 121 grams, which is not enough for whats has been asked.