Answer:
Manganese undergoes reduction
Oxygen undergoes oxidation
Explanations
What is oxidation?
3) Oxidation is defined as the loss of electron(s) of an atom or increase in the oxidation state of the atom while Reduction has to do with the gain of electron(s) by an atom and a reduction in the oxidation state of the atom.
Given the chemical reaction
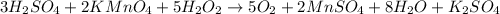
The half ionic reaction for the oxidation-reduction reaction is given as:
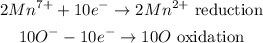
From the half-reaction, you can see there is a change in the oxidation state of Manganese from +7 to +2. This shows that Manganese undergoes reduction due to the reduction in its oxidation state.
For the oxygen element, there is a loss of election and an increase in its oxidation state from -1 to 0 showing that the oxygen element undergoes oxidation.
The same is not applicable for other e