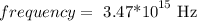 Step-by-step explanation
Step-by-step explanationfrequency is thhe number of waves that crosses a fixed point in one second . The unit of frequency is per second.
Step 1
a) convert the energy into joules
the equivalence is
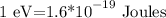
so, 5 eV, will be 5 times this amount, so
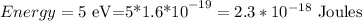
Step 2
we also know that, Photon energy(E) is the multiplication of plank's constant (h) and frequency(v)
so
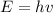
replace and solve for frequency(v)
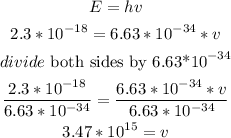
therefore, the answer is
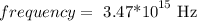
I hope this helps you