To find the percent by mass of oxygen we have to use the molar mass of oxygen and multiply it by 3 (number of atoms of O in Fe2O3). Then divide it by the molar mass of the compound (Fe2O3) and finally multiply it by 100, this way:
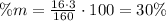
It means that the percent by mass of oxygen is 30%. The correct answer is (2) 30%.