As the questions says, we can calculate the percent composition of each element in a compound by dividing the molar mass of the corresponding element in the compound by the molar mass of the compound.
The molar masses of Cu and Cl are:
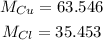
Thus, the molar masses of CuCl and CuCl₂ are:
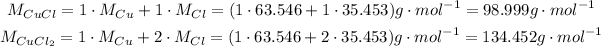
So, the percent compositions of copper and chlorine in CuCl are:
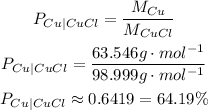
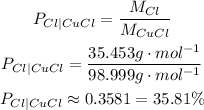
For CuCl₂, we need to consider two Cl, so:
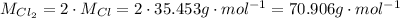
The percent compositions of copper and chlorine in CuCl₂ are:
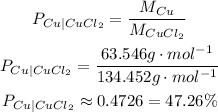

Answers:
For CuCl:
Percent composition of copper is approximately 64.19%.
Percent composition of chlorine is approximately 35.81%.
For CuCl₂:
Percent composition of copper is approximately 47.26%.
Percent composition of chlorine is approximately 52.74%.