Answer:
0.193 M of acid.
Step-by-step explanation:
To find the concentration of the acid, we're going to use the following formula:

Where C is concentration and V the volume. Subindex 1 can indicate the concentration and volume of the acid (HNO3) and subindex 2 can indicate the concentration and volume of the base (KOH).
In this case, we want to find C1, so let's clear the formula for this incongnite:
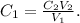
And finally, we replace the given data, like this:
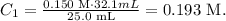
The concentration of acid would be 0.193 M.