Answer:
9 moles
Explanations:
Given the chemical reaction below;
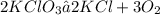
According to stoichiometry, 2 moles of KClO3 produces 3 moles of O2.
Given the following parameters
Moles of KClO3 = 6 moles
The moles of oxygen that is produced is given as;
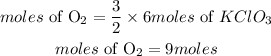
Hence the moles of O2 that will be produced is 9 moles