In order to calculate how much carbon-14 will be left after 17190 years, we can use the formula:
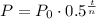
Where P0 is the final amount after t years, P0 is the initial amount and n is the period of half-life.
So, using P0 = 70, t = 17190 and n = 5730, we have:
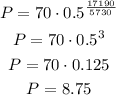
Therefore there will be 8.75 mg of carbon-14.