Answer: the heat necessary to change the temperature of 250g of water from 24 to 71C is 49.4 kJ.
Explanation:
The question requires us to determine the amount of heat energy necessary to heat 250g of water from 24 to 71°C, knowing that the heat capacity of water is 4.2 J/K.g.
We can use the following equation to calculate the heat energy of a sample:
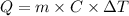
where Q is the heat energy we want to calculate, m is the mass of the sample, C is the heat capacity of the substance and ΔT is the temperature change (ΔT = T(final) - T(initial)).
Note that, as the heat capacity was given in units of Joules per Kelvin and grams, we'll need to adopt the following units of measurement:
- Q: it will be calculated in Joules (J);
- m: must be used in grams (g);
- ΔT: must be used in Kelvin (K).
However, note that as the temperature considered is the variation of temperature (or temperature change), if we calculate it in Celsius degrees or Kelvin, the value won't change:
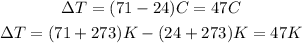
Therefore, we can apply the values m = 250g, C = 4.2 J/K.g ΔT = 47K to the equation shown above and calculate the heat energy (Q) as:
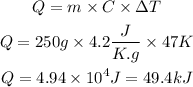
(note that 1 kJ = 1000 J, thus 4.94 x 10"4J = 49.4 kJ).
Therefore, the heat necessary to change the temperature of 250g of water from 24 to 71C is 49.4 kJ.