In order to determine the average kinetic energy, use the following formula:
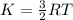
where,
R: gas constant = 8.314J/mol*K
T: temperature of the gas = 72.3+ 273 = 345.3K
Replace the previous values of the parameters into the formula for K:
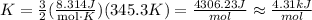
Hence, the average kinetic energy is approximately 4.31kJ/mol