In order to find the answer, let's analyze the formula below for the heat energy:
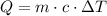
Where Q is the heat energy (in J), m is the mass, c is the specific heat and DeltaT is the change in temperature.
If the value of c is 10 times greater, then for the same amount of energy Q and the same mass m, the increase in temperature would be 10 times lower.
Therefore the correct option is b.