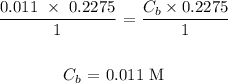Answer:
Step-by-step explanation:
Here, we want to answer some questions about titration
We shall be answering questions for sample 1 as follows
a) Mass of KHP in sample 1
That would be the difference between mass of beaker + KHP after first sample taken minus mass of beaker + KHP before sample taken
Mathematically, we have that as:
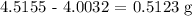
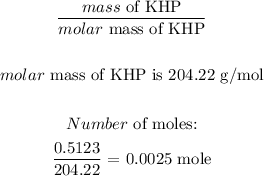
b) Moles of KHP
Mathematically, we have that as:
c) Volume of NaOH utilized
We can get that from the given table
We have the value as 22.75 mL/1000 = 0.2275 L
d) Molarity of NaOH from sample 1
Let us write the equation of reaction;
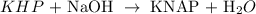
We have the general equation to use as:

But we need to calculate the concentration of the acid
We have the volume of acid used as 22.86 - 0.11 = 22.75 mL = 0.2275 L
The molarity would be:
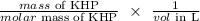
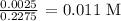
From the dilution equation above:
Ca is KHP molarity = 0.011 M
Va is KHP volume = 0.2275 L
Cb is NaOH molarity = ?
Vb is NaOH volume = 0.2275
na is the number of moles of acid in the balanced equation which is 1
nb is the number of moles of base is balanced equation which is 2
Substituting the values: