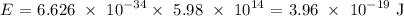Answer:
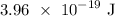
Step-by-step explanation:
Here, we want to get the energy in Joules
Given:
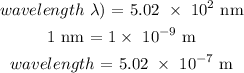
The frequency of light can be calculated using the formula as follows:
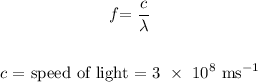
Calculating the frequency, we have it that:
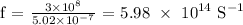
The Energy of a photon is calculated as:
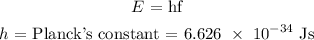
We proceed to multiply this with the frequency above as follows: