The first step is to convert all the measurements from mixed numbers to fractional numbers, this way:
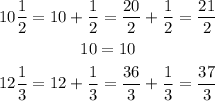
To find the number of cubic inches that are needed to fill the tank, find the volume of it.
The volume of a rectangular prism is given by the following formula:
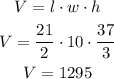
1295 cubic inches of water are needed to fill the tank.