The equation to calculate the slope is given to be:
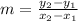
The following parameters are given:
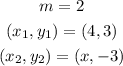
We can thereby substitute these values into the equation:
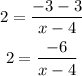
Cross multiplying, we have:
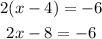
Adding 8 to both sides, we have:
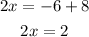
Dividing both sides by 2, we have:
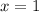
OPTION C is the correct answer.