Answer:
1) Workdone = -7.5 kJ
2) Energy = 23 kJ
Explanations:
The number of moles of the ideal gas, n = 3 moles
Temperature, T = 273K
Let the old volume be V₁
Let the new volume be V₂
The new volume is three times as large as the old volume
That is, V₂ = 3V₁
The gas constant, R = 8.314
1) The workdone in the gas
The workdone in expanding the gas is given by the formula:
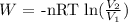
Substitute the values for this parameters into the formula above:
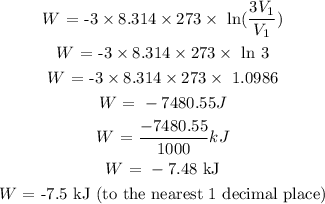
2) The energy required for a piston to compress the gas back to its original volume with an efficiency of 32%
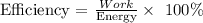
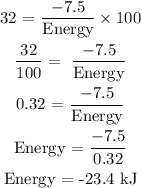
Since the piston is compressing the gas, a positive work is done on the system. Therefore, Energy = 23.4 kJ
Energy = 23 kJ (to the nearest whole number)