Answer:
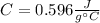
Step-by-step explanation:
Hello there!
In this case, according to the first law of thermodynamics it is possible to compute the gained heat by the water as shown below:
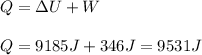
Thus, it is possible to solve for the specific heat of the gas as shown below:
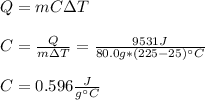
Best regards!