Given,
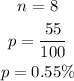
The probability mass function is calculated by the given formula,
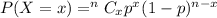
Here, x = 0, 1, 2, 3.....n.
Here, n = 8 and x = 5.
Calculate the probability that she gets exactly 5 bullseyes.
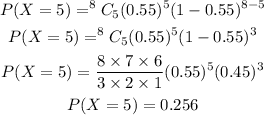
Therefore, the probability that she gets exactly 5 bullseyes is 0.256