The energy equation for closed systems is:
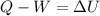
where:
• Q is the heat transfer to the system.
,
• W is the work done by the system.
,
• ΔU is the change of internal energy of the system.
In this case we know that the heat transfer is 63 J, the initial internal energy and final internal energy are 58 J and 93 J, respectively; plugging these values in the equation above and solving for the work we have:
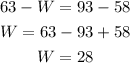
Therefore, the work done by the system is 28 J