Answer:
Option A is correct. 0.404g
Step-by-step explanation:
Given the chemical reaction between sodium and water as shown below:
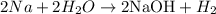
From the reaction, we can see that 2 moles of water molecules react to form 1 mole of the Hydrogen molecule. This shows that 0.400moles of water will react with sodium to form 0.200 moles of H2
Get the mass of hydrogen produced using the formula:
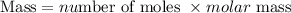
Since the molar mass of hydrogen is 2.016 g. The mass of hydrogen produced will be expressed as;
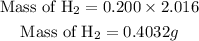
This shows that the mass of the hydrogen produced when 0.400 mol of water reacts completely is approximately 0.404g