Given: A equation-

Required: To determine the y coordinate when the x coordinate is -3.
Explanation: The given equation is-

Substituting the value of x=-3 into the equation as-
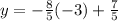
Further solving-
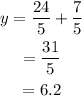
Final Answer: The value of the y coordinate is 6.2 or 31/5.