Answer
6.625 mL
Step-by-step explanation
Given:
Mass of manganese = 4.77 x 10¹⁰ ng
Density of manganese = 7.20 g/cm³
Volume of manganese = ?
The mass of the manganese given is in nanogram, ng and you need to be convert it to gram, g.
Conversion of ng to g
1 ng = 1.0 x10⁻⁹ g
∴ 4.77 x 10¹⁰ ng = 47.7 g
Hence, you can now calculate the volume of the manganese in mL using the formula below:
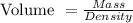
Volume of manganese = (47.7 g)/(7.2 gcm⁻³) = 6.625 mL