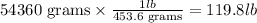WE just need to apply the formula of the density:
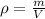
where p is the density, m is the mass and V is the volume, in this case they are asking about the mass so we have to solve that equation for the mass:
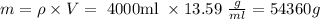
Please notice that to solve that question every thing has to be in the same units
To transform grams into pounds we need to know that 1 pound is 456.6 grams and use this information as conversion factor: