Answer
10 liters
Step-by-step explanation
We will assume the given percentages are those by volume (i.e. v/v).
We're asked to find the volume, in L, of a 30% v/v that must be added to 30 L of 70% solution v/v to obtain a solution that 60% v/v.
To do this, we can set up sort of an algebraic equation that represents this situation.
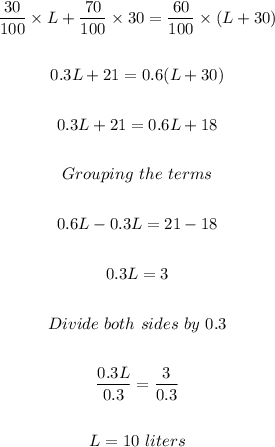
Therefore, 10 liters of 30% chemical solution must be mixed with 30 liters of 70% chemical solution to get a 60% mixture