The pOH and the pH are related by the following equation:
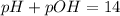
Thus, we can solve for pH and substitute the pOH = 5 to find the pH:
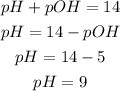
So, the pH is 9.
We can classify the solution by using the folowing:
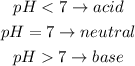
Since the pH is 9, it is greater than 7, so the solution is a base.