Explanation
Firstly, we need to write down the balanced chemical equation
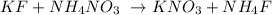
The next step is to write the state of each substance
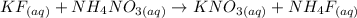
The next step is to split strong electrolytes into ion
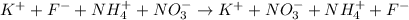
The next step is to cross out the spectator ions on both sides of the complete ionic equation
From the complete ionic equation above, you will see that K^+ appears on both sides,