You can realize that sulfuric acid (H2SO4) is a strong acid because it can dissociate completely in an aqueous solution, so we can assume that the concentration [H3O+] is 0.0084 M.
We can find [OH-], using the following formula:
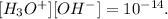
So, let's clear [OH-] and replace the data that we have:
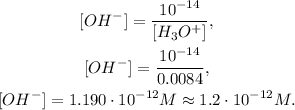
The answer is that we have 0.0084 M of [H3O+] and 1.2 x 10^(-12) M of [OH-].