Answer:
B and D.
Step-by-step explanation:
A reaction is correctly balanced when there is the same amount of each element in each side of the reaction.
The balanced reactions are:
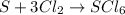
Because in each side of the reaction there are: 1S and 6 Cl.
And:
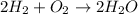
Because in each side of the reaction there are: 4H and 2O.