The first step we have to follow is to add the given mass to find the total mass of the solution:
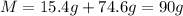
Now, divide the mass of the solute by the mass of the solution and multiply it times 100 to find the percent by mass:
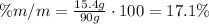
The percent by mass of the solute in the solution is 17.1%.