"45.5% by mass NaF solution" means that 45.5% of the mass of the solution is NaF.
The solution has 34.2g of mass, so 45.5% of this is the mass of NaF:
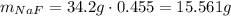
To transform this into moles, we need the molar mass of NaF, which we can calculate using the atomic masses of Na and F:
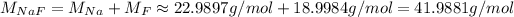
So, the number of moles is:
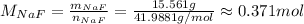
So, there are approximately 0.371 mol of NaF.