To find the mass of oxygen we have to use the ideal gas law to find the number of moles of gas at the given conditions.
First, convert the pressure in torr to atm:
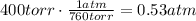
Use the ideal gas law and solve it for n:
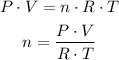
Replace for the known values (R, the ideal gas constant, has a value of 0.082atmL/molK):
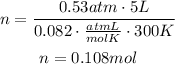
Finally use the molecular mass of oxygen gas to find the mass of 0.108 moles of the gas:
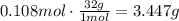
5 liters of O2 at 300K and 400torr will weigh 3.447154g.