In this problem, we can apply ratios to find the amount of boric acid in the solution.
We know that the ratio of boric acid to distilled water is 1:25. So, we can set up a proportion:
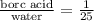
Since we have a total of 900 mL of water, we can add the value of water and let x represent the boric acid:
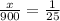
Next, we can solve the proportion by cross multiplying:
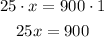
Finally, we can divide by 25:
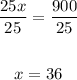
So, there are 36mL of boric acid.