Answer
0.18 moles of gas
Step-by-step explanation
Given:
Temperature, T = -13 °C = (-13 + 273.15 K) = 260.15 K
Pressure, P = 1.5 atm
Volume, V = 2.5 L
What to find:
The number of moles of gas the tire hold.
Step-by-step solution:
The number of moles, n of gas the tire hold can be determined using the ideal gas equation.
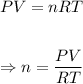
Plugging the values of the given parameters and R = 0.0821 atm•L/mol•K into the ideal gas equation;
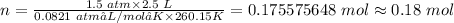
The number of moles of gas the tire would hold is 0.18 moles